What is the purity of L-ergothioneine powder?
l ergothioneine powder is a powerful antioxidant compound that has gained significant attention in the health and wellness industry. As more people become interested in its potential benefits, questions about its purity and quality have become increasingly important. In this article, we'll explore the purity of L ergothioneine powder, how it's determined, and why it matters for those seeking to incorporate this supplement into their health regimen.
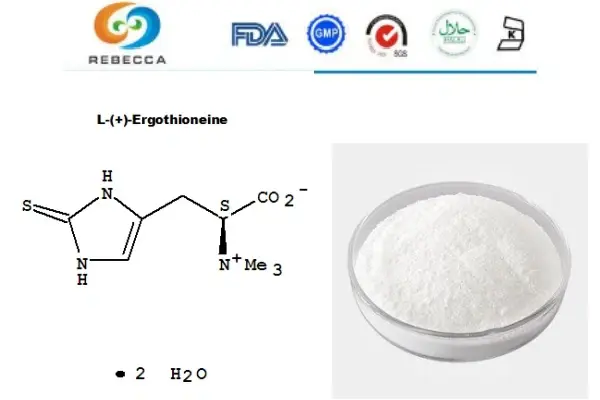
How is the purity of L-ergothioneine powder determined?
The purity of L ergothioneine powder is typically determined through a series of sophisticated analytical techniques. These methods ensure that the powder contains the highest possible concentration of the active compound while minimizing the presence of impurities or contaminants.
One of the primary methods used to assess purity is High-Performance Liquid Chromatography (HPLC). This technique separates the components of a mixture based on their chemical properties, allowing scientists to identify and quantify the amount of L-ergothioneine present in a sample. HPLC can detect even minute quantities of impurities, making it an invaluable tool in quality control.
Another method commonly employed is Mass Spectrometry (MS). This analytical technique measures the mass-to-charge ratio of ions, providing detailed information about the molecular structure and composition of the L ergothioneine powder. MS can identify any unexpected compounds or contaminants that may be present in the sample.
Nuclear Magnetic Resonance (NMR) spectroscopy is also utilized to verify the purity and structure of L-ergothioneine. This technique provides detailed information about the molecular structure of the compound, allowing researchers to confirm its identity and detect any structural anomalies.
In addition to these advanced analytical methods, manufacturers may also conduct elemental analysis to determine the exact composition of the powder. This helps ensure that the product meets the expected ratios of carbon, hydrogen, nitrogen, and sulfur characteristic of pure L-ergothioneine.
It's worth noting that reputable manufacturers often employ a combination of these techniques to provide a comprehensive assessment of their product's purity. This multi-faceted approach helps ensure that the L-ergothioneine powder meets the highest standards of quality and purity.

Why is purity important for L-ergothioneine supplements?
The purity of L ergothioneine powder is crucial for several reasons, all of which directly impact the safety, efficacy, and overall quality of the supplement.
Firstly, purity directly correlates with potency. A higher purity level means that a greater percentage of the powder is composed of actual L-ergothioneine molecules. This translates to a more potent product, potentially offering greater benefits to the user. When consumers purchase L-ergothioneine supplements, they're investing in the compound's unique properties, and a high-purity product ensures they're getting the most value for their money.
Secondly, purity is intrinsically linked to safety. Impurities in dietary supplements can potentially cause adverse reactions or interfere with the intended effects of the active compound. By ensuring a high level of purity, manufacturers minimize the risk of unexpected side effects or interactions that could arise from contaminants or byproducts of the production process.
Moreover, consistent purity levels are essential for reliable dosing. When healthcare professionals or researchers recommend specific doses of L-ergothioneine, their recommendations are based on studies using pure compounds. If a supplement's purity varies significantly, it becomes challenging to achieve the intended dosage, potentially impacting the supplement's effectiveness.
Purity also plays a role in the stability and shelf life of the product. Pure L-ergothioneine is generally more stable and less prone to degradation over time. This means that high-purity powders are more likely to maintain their potency throughout their shelf life, ensuring that consumers receive the full benefits of the supplement even after extended storage.
Lastly, the purity of L-ergothioneine powder is a reflection of the manufacturer's commitment to quality. Companies that invest in producing high-purity products often have stringent quality control measures in place throughout their production process. This attention to detail typically extends to other aspects of their operations, suggesting a higher overall quality of products and services.

What are the purity standards for L-ergothioneine powder?
The purity standards for L ergothioneine powder can vary depending on the intended use and the regulatory environment. However, in the supplement industry, there are generally accepted benchmarks that manufacturers strive to meet or exceed.
For pharmaceutical-grade L-ergothioneine, which is used in clinical studies and high-end supplements, the purity standard is typically set at 99% or higher. This extremely high level of purity ensures that the powder contains virtually no impurities or contaminants, making it suitable for the most demanding applications.
In the nutraceutical and dietary supplement industry, many reputable manufacturers aim for a purity of 98% or higher for their pure l ergothioneine. This level of purity is considered excellent for consumer-grade supplements and is often sufficient to provide the intended health benefits.
It's important to note that these purity levels refer to the active L-ergothioneine content of the powder. The remaining small percentage may consist of trace amounts of moisture, residual solvents used in the purification process, or other benign compounds that do not affect the safety or efficacy of the product.
Some manufacturers may offer L-ergothioneine powders with purity levels between 95% and 98%. While these products can still be effective, they may require slightly higher doses to achieve the same results as higher-purity options.

In addition to purity standards, there are other quality parameters that reputable manufacturers adhere to. These may include:
- Heavy metal testing to ensure the powder is free from harmful contaminants like lead, mercury, or arsenic.
- Microbial testing to confirm the absence of harmful bacteria, yeasts, or molds.
- Residual solvent testing to verify that any solvents used in the production process have been effectively removed.
- Particle size analysis to ensure consistent powder properties and dissolution characteristics.
It's worth noting that while these purity standards are widely recognized in the industry, there isn't a single, universally mandated standard for L-ergothioneine powder purity. This is why it's crucial for consumers to purchase from reputable manufacturers who are transparent about their quality control processes and can provide certificates of analysis for their products.
Some regulatory bodies, such as the European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration (FDA), have reviewed the safety of L-ergothioneine for use in dietary supplements. While they don't set specific purity standards, their approval processes consider the overall quality and safety of the ingredient, including its purity.
As research continues to uncover the potential benefits of L-ergothioneine, it's likely that purity standards will continue to evolve. Manufacturers committed to producing high-quality L ergothioneine powder will need to stay abreast of these developments and potentially adapt their production and quality control processes accordingly.
Ready to experience the benefits of high-purity pure l ergothioneine? Contact Rebecca Bio-Tech, a leading manufacturer of premium L-ergothioneine products. For more information or to request samples, please email us at information@sxrebecca.com. Our team of experts is ready to assist you with your L-ergothioneine needs and answer any questions you may have about our high-quality products.
References:
- Halliwell, B., Cheah, I. K., & Tang, R. M. Y. (2018). Ergothioneine - a diet-derived antioxidant with therapeutic potential. FEBS Letters, 592(20), 3357-3366.
- Cheah, I. K., & Halliwell, B. (2012). Ergothioneine; antioxidant potential, physiological function and role in disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1822(5), 784-793.
- Ey, J., Schömig, E., & Taubert, D. (2007). Dietary sources and antioxidant effects of ergothioneine. Journal of Agricultural and Food Chemistry, 55(16), 6466-6474.
- Gruber, J., Fong, S., Chen, C. B., Yoong, S., Pastorin, G., Schaffer, S., ... & Halliwell, B. (2013). Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnology Advances, 31(5), 563-592.
- Servillo, L., D'Onofrio, N., & Balestrieri, M. L. (2017). Ergothioneine antioxidant function: from chemistry to cardiovascular therapeutic potential. Journal of Cardiovascular Pharmacology, 69(4), 183-191.
- Pfeiffer, C., Bauer, T., Surek, B., Schömig, E., & Gründemann, D. (2011). Cyanobacteria produce high levels of ergothioneine. Food Chemistry, 129(4), 1766-1769.








