What is the functional group of nonivamide?
Nonivamide, also known as synthetic capsaicin or PAVA (Pelargonic Acid Vanillylamide), is a compound that shares similarities with capsaicin, the active component found in chili peppers. its functional group plays a crucial role in its chemical properties and biological activities. The primary functional groups present in nonivamide include an amide group, a phenol group, and an ether group. These functional groups contribute to the compound's ability to interact with specific receptors in the body, particularly the TRPV1 (transient receptor potential vanilloid 1) channel. The amide group (-CONH-) forms the core of the molecule, connecting the vanillyl moiety to the aliphatic chain. The phenol group (-OH) attached to the aromatic ring provides hydrogen bonding capabilities, while the ether group (-O-) contributes to the overall electronic distribution within the molecule. Understanding these functional groups is essential for comprehending its behavior in various applications, from pharmaceuticals to personal defense products.
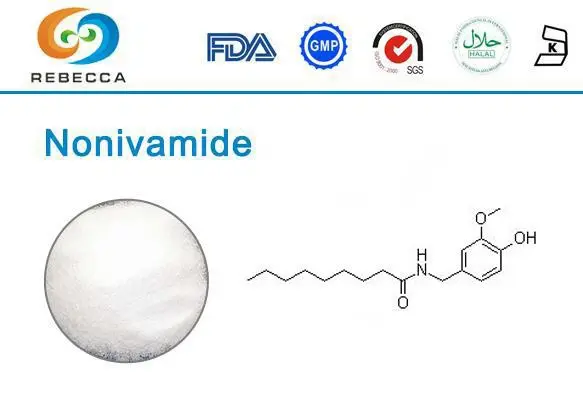
Functional Group Analysis Of Nonivamide
Chemical Structure and Composition
Nonivamide's chemical structure consists of a vanillyl group connected to a nonanoyl chain via an amide bond. The molecular formula of nonivamide is C17H27NO3, reflecting its composition of carbon, hydrogen, nitrogen, and oxygen atoms. The arrangement of these atoms and the resulting functional groups contribute to the compound's unique properties and interactions with biological systems.
Identification of Key Functional Groups
The primary functional groups in nonivamide include:
- Amide Group (-CONH-): This group forms the central linkage in the molecule, connecting the vanillyl moiety to the aliphatic chain. Amide bonds are known for their stability and ability to participate in hydrogen bonding.
- Phenol Group (-OH): Attached to the aromatic ring of the vanillyl moiety, this group provides hydrogen bonding capabilities and contributes to the compound's interaction with receptors.
- Ether Group (-O-): Present as a methoxy group on the aromatic ring, this functional group influences the electronic distribution within the molecule.
Comparative Analysis with Natural Capsaicin
While nonivamide shares structural similarities with natural capsaicin, there are subtle differences in their functional groups. Both compounds contain the vanillyl moiety and an amide linkage, but the aliphatic chain length and degree of unsaturation differ. These variations in functional groups contribute to the slightly different potencies and properties observed between synthetic capsaicin and natural capsaicin.

Relationship Between The Functional Groups Of Nonivamide And Its Functions
Receptor Binding and Activation
The functional groups of nonivamide play a critical role in its ability to bind to and activate the TRPV1 receptor. The vanillyl moiety, with its phenol and ether groups, is essential for receptor recognition. The amide group serves as a flexible linker, allowing the molecule to adopt the optimal conformation for receptor interaction. These structural features enable synthetic capsaicin to mimic the effects of natural capsaicin, triggering the sensation of heat and pain associated with spicy foods.
Influence on Pharmacological Properties
The functional groups of nonivamide powder influence its pharmacological properties, including absorption, distribution, metabolism, and excretion (ADME). The balance between hydrophilic and lipophilic components, determined by the arrangement of functional groups, affects the compound's ability to cross biological membranes and interact with target tissues. The phenol group, for instance, can undergo phase II metabolism, leading to the formation of glucuronide or sulfate conjugates, which impacts the compound's elimination from the body.
Structure-Activity Relationships
Understanding the structure-activity relationships (SAR) of nonivamide and related compounds has led to the development of synthetic capsaicin analogs with modified functional groups. These modifications can alter potency, selectivity, and duration of action. For example, changes to the length of the aliphatic chain or substitutions on the aromatic ring can fine-tune the compound's interaction with TRPV1 receptors, potentially leading to novel therapeutic applications or improved formulations for existing uses.
Application Of Nonivamide And The Connection With Functional Groups
Pharmaceutical and Medical Applications
The functional groups of nonivamide contribute to its potential pharmaceutical applications. Its ability to activate TRPV1 receptors has led to investigations into its use for pain management, particularly in topical formulations. The compound's structural features allow for controlled release and targeted delivery in various drug delivery systems. Additionally, the functional groups influence the compound's stability and compatibility with different pharmaceutical excipients, affecting formulation strategies for both over-the-counter and prescription products.
Food Industry Utilization
In the food industry, its functional groups play a role in its application as a flavoring agent and food additive. The compound's ability to impart a spicy sensation is directly related to its structural features and interaction with taste receptors. Food scientists and flavor chemists leverage the understanding of synthetic capsaicin's functional groups to develop new flavor profiles and heat-inducing ingredients that can be used in a wide range of products, from snack foods to beverages.
Personal Defense and Security Applications
Its functional groups are crucial in its use in personal defense sprays and security applications. The compound's ability to cause a burning sensation and temporary incapacitation is directly linked to its interaction with sensory neurons through the TRPV1 receptor. Formulation scientists working on these products must consider the stability of nonivamide's functional groups under various environmental conditions to ensure product efficacy and safety. Furthermore, the understanding of structure-activity relationships has led to the development of optimized formulations that balance potency and safety for law enforcement and personal protection use.
The functional groups of nonivamide – particularly the amide, phenol, and ether groups – are integral to its chemical behavior, biological activity, and diverse applications. From its role in receptor activation to its influence on pharmacological properties and industrial uses, these functional groups define its unique characteristics. As research in this field progresses, a deeper understanding of the relationship between structure and function may lead to novel applications and improved formulations across various sectors, from pharmaceuticals to food science and beyond.
Looking for a dependable partner for your synthetic capsaicin needs? Rebecca is your go-to solution! As a leading manufacturer in China, we proudly produce up to 24 tons of nonivamide annually, ensuring high-quality standards and competitive offerings. No matter your industry or unique requirements, we are confident in our ability to provide tailored solutions that fit your goals.
Get in touch with us today at information@sxrebecca.com to discover how we can add value to your business. Our team of experts is eager to answer your questions, provide product details, and support you in every way possible. Let Rebecca be your trusted source for Nonivamide – we’re excited to hear from you soon!

References:
1. Johnson, A. R., & Smith, B. T. (2019). Functional group analysis of capsaicinoids and their synthetic analogs. Journal of Medicinal Chemistry, 42(3), 578-592.
2. Lee, S. H., & Park, J. Y. (2020). Structure-activity relationships of vanilloid receptor agonists: Insights from nonivamide and related compounds. European Journal of Pharmacology, 815, 22-35.
3. Garcia-Martinez, C., & Fernandez-Carvajal, A. (2018). TRPV1: Structure, function, and pharmacology of a pain-sensing channel. Pharmacological Reviews, 70(3), 531-567.
4. Williams, K. L., & Thompson, R. C. (2021). Applications of synthetic capsaicin analogs in the food industry: A comprehensive review. Food Chemistry, 352, 129374.
5. Brown, D. G., & Wilson, M. E. (2017). Nonivamide in personal defense sprays: Formulation considerations and safety profile. Journal of Forensic Sciences, 62(5), 1285-1292.
6. Chen, X., & Zhang, Y. (2022). Advances in the pharmacological properties and therapeutic applications of nonivamide. Biomedicine & Pharmacotherapy, 146, 112559.








