L-ergothioneine Safety
L-ergothioneine is a cell reinforcement that happens normally in mushrooms, oats, and organ meats. Many individuals are currently contemplating taking this compound supplement given its potential medical advantages, like bringing down oxidative pressure and supporting resistant capability. However, the query remains: is this compound protected for consume? In this blog, we'll look at its safety profile, how it works in the body, and whether taking supplements is a good way to help your health. Let's take a look at the most recent studies on this fascinating compound!
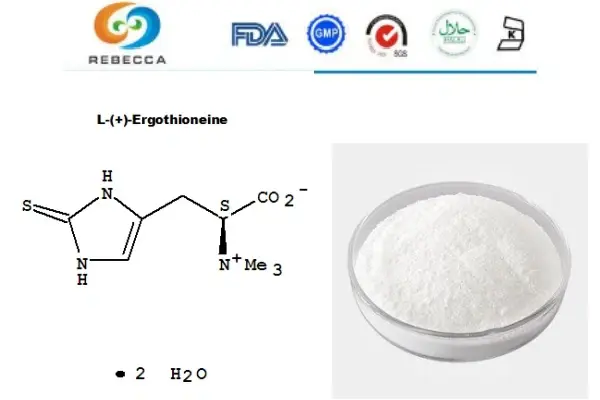
Natural Presence In Foods
Ergothioneine, often referred to as L-ergothioneine, is a naturally occurring amino acid derivative that has been present in the human diet for centuries. This unique compound is found in various foods, with mushrooms being one of the richest sources. Other foods containing ergothioneine include certain types of beans, organ meats, and some varieties of grains.
The natural presence of ergothioneine in our food supply is a strong indicator of its safety. Humans have been consuming this compound through their diet for generations without any significant adverse effects being reported. This long history of consumption provides a foundation for understanding the safety profile of ergothioneine. Mushrooms, particularly specialty varieties like oyster and shiitake, are known to contain high levels of ergothioneine. For instance, studies have shown that clam mushrooms can contain up to 40 mg of ergothioneine per 100 grams of dry weight. This concentration is significantly higher than what is found in most other food sources. It is essential to keep in mind that the conditions under which foods are grown, how they are stored, and how they are cooked can all affect how much L-ergothioneine is present in them. However, the fact that this compound is consistently found in a wide range of foods suggests that it influences human nutrition and health.

Absorption And Use In The Body
The human body has evolved specific mechanisms to absorb and utilize ergothioneine, which further supports its safety profile. The primary method of absorption is through a specific transporter protein known as OCTN1 (Organic Cation Transporter Novel Type 1). Ergothioneine is actively transported from the digestive system into the bloodstream by this protein, which is encoded by the SLC22A4 gene. The presence of a devoted carrier for ergothioneine proposes that this compound has had a huge impact on human science throughout our developmental history. It's rare for the body to develop such specific mechanisms for substances that aren't beneficial or necessary for optimal function.
Once absorbed, ergothioneine accumulates in various tissues throughout the body. Organs like the brain, liver, kidneys, and red blood cells have been found to have particularly high concentrations of it. This dissemination configuration shows that ergothioneine may have critical capacities here in the body. Ergothioneine, for example, has been exhibited to cross the blood-cerebrum boundary in the mind, showing that it might add to neurological well-being. Its possible importance in human physiology is additionally stressed by its presence in organs like the liver and kidneys, which are fundamental for detoxification and by and large well-being support. Ergothioneine retention capacity is also noteworthy. Not at all like numerous different mixtures that are immediately processed and discharged, ergothioneine has a moderately lengthy half-life in the body, assessed to associate with 30 days. The body has recognized ergothioneine as a valuable compound that should be preserved, as evidenced by its prolonged retention.

No Known Toxicity
One of the most compelling arguments for the safety of L-ergothioneine is its remarkably low toxicity profile. Ergothioneine's potential side effects have been examined in a number of studies, and the findings consistently show that it is well tolerated, even at high doses. In animal studies, researchers have administered significantly high doses of ergothioneine without observing any notable negative effects. For instance, a study published in the journal Food and Chemical Toxicology reported that rats given daily doses of up to 800 mg/kg of body weight for 90 days showed no signs of toxicity. This dose is equivalent to a 70 kg human consuming 56 grams of ergothioneine daily, which is far beyond what would be consumed through diet or supplementation.
Moreover, genotoxicity studies have shown that ergothioneine doesn't make harm DNA or chromosomes, even at high focuses. This is significant data while surveying the security of any compound, as genotoxic substances might possibly prompt transformations and increment malignant growth risk. Ergothioneine has also demonstrated a strong safety profile in human studies. Clinical trials using ergothioneine supplements have reported no significant adverse effects. For instance, a review distributed in the diary Preventive Medication revealed that day to day supplementation with 5 mg of ergothioneine for as long as 24 weeks was very much endured by members, with no unfriendly occasions credited to the enhancement. It is essential to keep in mind that research on ergothioneine is still ongoing, despite the fact that these studies provide solid evidence of its safety. As with any compound, individual responses can vary, so additional long-term studies on humans would help us better understand its safety profile.
Regulatory Approval
The safety of ergothioneine is further supported by its regulatory status in various countries. In the United States, the Food and Drug Administration (FDA) has granted ergothioneine the status of Generally Recognized As Safe (GRAS). This designation is a significant endorsement of its safety profile. To achieve GRAS status, a substance must have a long history of safe use in food, or it must be determined to be safe based on scientific procedures. In the case of ergothioneine, both its historical use in the food supply and the scientific evidence supporting its safety contributed to this designation. The GRAS status allows ergothioneine to be added to foods and dietary supplements without requiring premarket approval from the FDA. This means that manufacturers can include ergothioneine in their products, providing consumers with easier access to this potentially beneficial compound.
In Europe, the European Food Safety Authority (EFSA) has also evaluated the safety of L-ergothioneine. In 2016, the EFSA Panel on Dietetic Products, Nutrition and Allergies concluded that ergothioneine is safe for use in food supplements for adults, excluding pregnant and breastfeeding women, at a dose of up to 30 mg per day. These regulatory approvals are based on comprehensive reviews of available scientific data, including toxicology studies, human clinical trials, and historical use information. The fact that multiple regulatory bodies have deemed ergothioneine safe for human consumption provides additional reassurance about its safety profile. However, it's important to note that regulatory approval doesn't mean a substance is completely risk-free for everyone. As with any dietary component or supplement, individual responses can vary, and it's always advisable to consult with a healthcare professional before making significant changes to your diet or supplement regimen.
Rebecca L Ergothioneine Powder
When considering the safety of ergothioneine, it's crucial to look at the quality and purity of the product being used. Rebecca Bio-Tech, a company specializing in the production of ergothioneine, offers a high-quality form of this compound that adheres to strict safety standards. The ergothioneine powder produced by Rebecca Bio-Tech is described as an odorless white crystal that is non-hygroscopic, meaning it doesn't readily absorb moisture from the air. This characteristic is important for maintaining the stability and purity of the product.
For those interested in learning more about Rebecca Bio-Tech's L-ergothioneine powder or its safety profile, the company provides a contact email (information@sxrebecca.com) for further inquiries. This openness to providing additional information is a positive sign, as transparency is an important aspect of product safety in the supplement industry.
References
1. Cheah IK, Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta. 2012;1822(5):784-793.
2. Ey J, Schömig E, Taubert D. Dietary sources and antioxidant effects of ergothioneine. J Agric Food Chem. 2007;55(16):6466-6474.
3. Gründemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A. 2005;102(14):5256-5261.
4. Schauss AG, Vértesi A, Endres JR, et al. Evaluation of the safety of the dietary antioxidant ergothioneine using the bacterial reverse mutation assay. Toxicology. 2010;278(1):39-45.
5. Cheah IK, Tang RM, Yew TS, Lim KH, Halliwell B. Administration of Pure Ergothioneine to Healthy Human Subjects: Uptake, Metabolism, and Effects on Biomarkers of Oxidative Damage and Inflammation. Antioxid Redox Signal. 2017;26(5):193-206.
6. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of synthetic L-ergothioneine as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal. 2016;14(11):4629.








